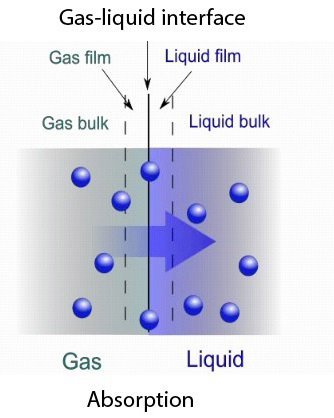
In scientific terms, absorption refers to the process by which a substance takes in another substance. Absorption can be achieved through a variety of mechanisms, such as chemical reactions, diffusion, and osmosis.
In chemical reactions, absorption occurs when one substance is taken up by another, resulting in a chemical change. For example, when iron oxide (Fe2O3) is heated, it can absorb carbon monoxide (CO) to form iron carbide (Fe3C):
Fe2O3 + 3CO -> 2Fe3C + 3CO2
Substances absorb when they move to an area of low concentration from an area of high concentration in diffusion. This can happen through a variety of mechanisms, such as simple diffusion, facilitated diffusion, or active transport. For example, when a solution of salt water is placed in a container with a membrane that is permeable to water but not to salt, the water will move through the membrane from the saltwater solution into the container, resulting in the absorption of the water by the membrane.
The process of osmosis involves the movement of water through a semipermeable membrane. During this process, water moves to an area of low concentration from an area of high concentration, resulting in its absorption by the membrane.
Absorption is a common process that occurs in many different contexts, from chemical reactions to the movement of substances across biological membranes. In fields such as chemistry, biology, and physics, it is an important concept.