Introduction
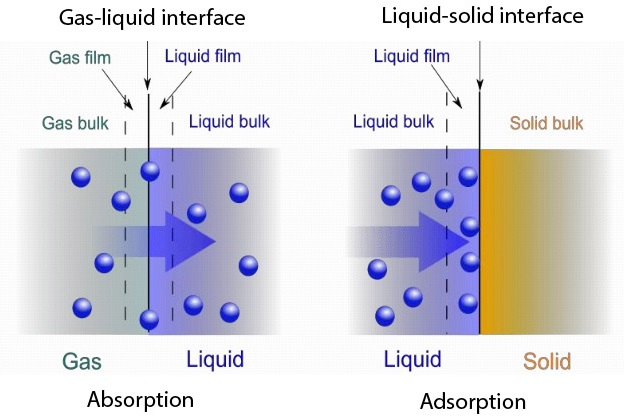
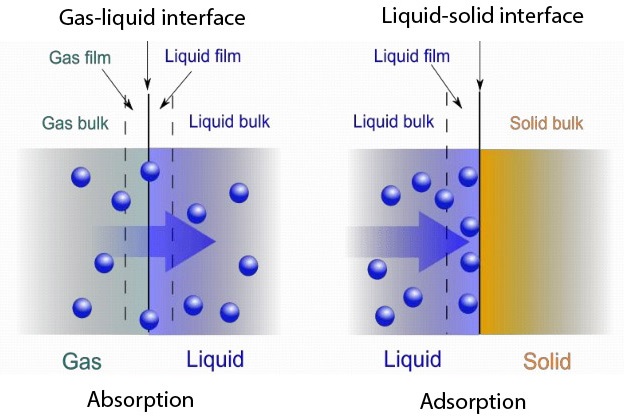
Absorption & Adsorption are two major unit operations that have their own differences. Both processes have the ability to extract or catchup solutes from one to another. There are quite a few differences between the processes. In general, absorption will result in penetrating a substance from a solution to another substance. Here the substance will enter into another substance via diffusion and will remain inside the latter. Whereas in Adsorption the substance from the solution will only attach to the surface of another substance.
Absorption
Here the substance that will penetrate is defined as absorbate and the substance that takes the absorbate is called absorbent. For an instance will consider the scrubbing process. Gas streams from fuel burns tend to carry certain impurities. Hence these gas streams must be treated before discharging them into the atmosphere. We will assume that a certain gas stream carries Sulpher Dioxide. Here the absorbate is Sulphur Dioxide. When the gas stream goes past a water tank, Sulphur Dioxide will dissolve in the water. Here water becomes the absorbant. The gas stream coming out of the water will not carry Sulphur Dioxide along with it.
Adsorption
Here a material containing a certain mixture will adhere to the surface of another substance. This helps to remove the substance from the initial solution.
As an example, we will consider the water purification process through activated carbon. Water may contain ample amounts of organic matter. Some of these organic matters might not be ideal for drinking purposes. Therefore in most cases, activated carbon beds are used to overcome the issue.
Here Activated carbon particles will be the Adsorbent. Organic matter containing in the water will be the adsorbate. When the water flows through the carbon bed organic matter will stick to the porous structure of the carbon particles. Hence water stream coming out of the bed will have less organic matter.
Difference between Absorption & Adsorption
There are few notable differences in the processes. We will look at few facts on both processes to differentiate.
| Fact | Absorption | Adsorption |
| Basic Introduction | Is the unit operation of diffusing a substance from a solution to another substance | Is the unit operation of sticking a substance on a surface of another substance. |
| Occurrence | Here the absorbate particles will sneak into the absorbent | Here the adsorbate particles will only attach to the surface of the adsorbent |
| Concept | Absorbate will diffuse to the absorbent depending on the size of the particle, the ability to extract the substance by the absorbent, and the concentration of the absorbate in the two phases. | Adsorbate will stick to the adsorbent depending on the surface attraction of each substances, porosity of the adsorbent and the capacity of the adsorbent. |
| Heat generation | This is an endothermic Reaction | This is a exothermic reaction |
| Rate of process | Occurs at a consistent rate throughout its operation | Will keep increasing till the operation reaches its equilibrium |
| Distribution of the substance | Absorbate will distribute evenly throughout the absorbent | Adsorbate concentration will be much higher in the surface of the adsorbent |
| Interaction | Once the substance enters the absorbent both will act as a solution and there won’t be any reactions either. Virtually they will act as a mixture. | Adsorbate will stick to the adsorbent in the form of Van der Wall’s or Covalent bonds |
| Seperation | Absorbate has to be separated into different phases using chemical interactions. | A regeneration process will remove the adsorbate and the newly introduced regeneration substance will stick to the substance |