Enthalpy & Entropy are two crucial factors in thermodynamics. Enthalpy reveals the total heat of a closed system. Its unit is KJ/mol. whereas Entropy provides an idea of the randomness of a closed system. The unit of entropy is KJ/K. Simply, Entropy is the Enthalpy difference of a system per Kelvin or Celcius. Enthalpy & Entropy have some kind of differences. Still, there is an important relationship between the two facts. Therefore, combinations of these factors are vital in describing a system.
Category Archives: Energy
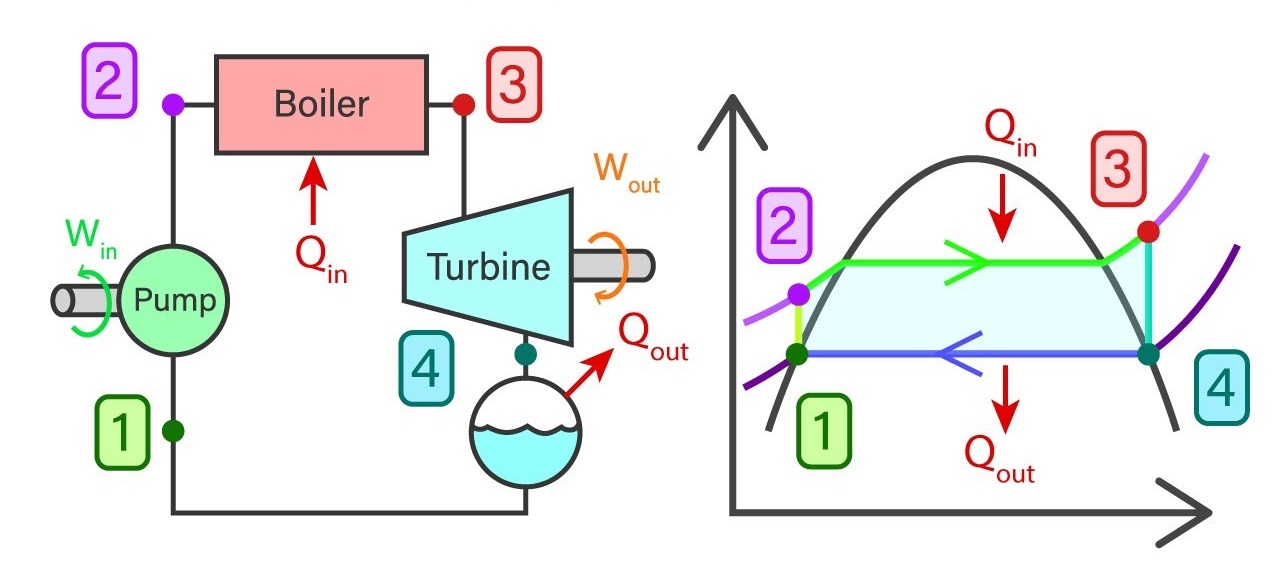
Introduction The Rankine cycle has become the goto process in the vapor power plants. In contrast to the Carnot cycle, this method of power generation provides more value to the outcome of the process. Its ability to increase the working temperature, providing high-quality steam with less moisture content to the turbine, and much easier handling […]
Renewable energy can be defined as the energy obtained from natural sources or processes that are replenished continuously. Thus, renewable energy resources are not going to deplete after usage.